Red Light Therapy for Arthritis Pain Relief
Red Light Therapy for Arthritis Pain Relief: A Science-Backed Solution You Can Use at Home
Summary:
Reduces inflammation and oxidative stress, easing joint pain in osteoarthritis and rheumatoid arthritis
Alleviates pain and stiffness, including morning stiffness in RA
Improves joint function, enhancing grip strength, mobility, and overall physical performance
Stimulates cartilage repair, promoting chondrocyte activity and mesenchymal stem cell differentiation
Enhances microcirculation, increasing blood flow to joints and reducing ischemia-related pain
Supports non-pharmacologic management, suitable for those seeking alternatives to NSAIDs and opioids
Safe, non-invasive, and drug-free, with minimal side effects like mild warmth or transient redness
Accessible treatment, with at-home LED devices offering convenient arthritis relief options
Introduction
Arthritis affects over 58 million Americans, causing chronic pain, stiffness, and reduced mobility. For many, daily activities like walking, climbing stairs, or even opening a jar can feel overwhelming. While medications and injections can help, they often come with side effects and don’t address long-term healing.
That’s where Red Light Therapy (RLT) — also known as photobiomodulation — comes in. Backed by clinical research, RLT offers a safe, drug-free way to reduce arthritis pain, improve joint function, and enhance quality of life. Even better, you can now bring the same powerful therapy used in clinics into your home with an FDA-cleared red light therapy panel.
👉 Want lasting relief without side effects? Shop our red light therapy panels for arthritis today.
What is Red Light Therapy?
Red light therapy uses red (660nm) and near-infrared (850nm) wavelengths to penetrate the skin and stimulate cells. By boosting mitochondrial energy (ATP) production, RLT helps reduce inflammation, repair tissues, and restore mobility.
How Red Light Therapy Helps Arthritis Pain
1. Reduces Joint Pain and Stiffness
A study in the Journal of the American Geriatrics Society found that patients using RLT experienced significant reduction in pain and morning stiffness compared to placebo groups.
By reducing inflammation, RLT helps arthritis sufferers move more freely.
👉 Struggling with stiff joints? Order your RLT panel today.
2. Improves Mobility and Range of Motion
RLT promotes collagen production and supports cartilage health, which can improve joint flexibility.
A clinical trial reported improved knee joint mobility in osteoarthritis patients after consistent red light therapy use.
3. Reduces Inflammation at the Cellular Level
Arthritis pain is largely driven by inflammation.
RLT reduces pro-inflammatory cytokines, calming joint inflammation naturally.
This makes it a long-term solution, not just a quick fix.
4. Supports Long-Term Joint Health
Regular use of RLT may help slow arthritis progression by improving circulation and tissue repair.
This means fewer flare-ups and better quality of life over time.
👉 Pro tip: Use your red light therapy panel daily for consistent results.
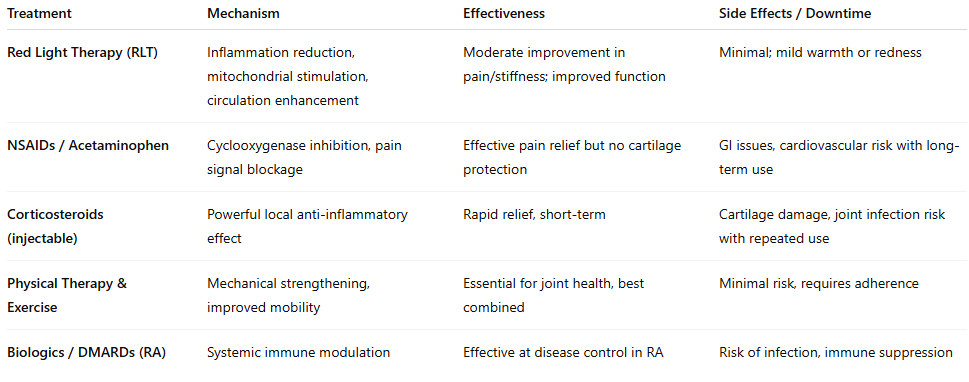
Table: Comparison of Arthritis Treatments
Why Choose At-Home Red Light Therapy Panels?
Clinic-based treatments can cost hundreds of dollars per session. With an at-home panel, you’ll get the same therapeutic benefits — for a fraction of the long-term cost.
✅ One-time purchase — unlimited sessions
✅ Easy to use — just 10–20 minutes per day
✅ FDA-cleared wavelengths proven for pain relief
✅ Safe & non-invasive — no drugs, no downtime
💬 “After using my red light therapy panel for 6 weeks, my arthritis pain has dropped by half. I can finally garden again without constant discomfort!” -Marty L. from White City, OR
👉 Ready for the same relief? Shop our panels now.
How to Use a Red Light Therapy Panel for Arthritis Relief
Distance: 6–12 inches from the affected joint
Session length: 15–20 minutes
Frequency: 4–5 times per week for best results
Best areas: knees, hands, hips, shoulders
FAQs: Red Light Therapy for Arthritis Pain
Q: How quickly will I see results?
Many people report reduced pain within 2–3 weeks, with maximum benefits after 8–10 weeks of consistent use.
Q: Is it safe for seniors?
Yes — RLT is gentle, safe, and well-tolerated across all ages.
Q: Can I use it with my current arthritis medications?
Absolutely. RLT is drug-free and may even help reduce your reliance on pain medication over time.
Why Buy From Medford Red Light Therapy?
💡 Expert-selected panels designed for arthritis pain relief
⚡ High power density for deeper joint penetration
🛡️ 30-day satisfaction guarantee
🚚 Fast shipping across the U.S.
👉 Don’t wait — your pain-free life starts today. Order your red light therapy panel now.
Conclusion & Next Steps
Arthritis pain doesn’t have to control your life. With red light therapy, you can reduce pain, improve mobility, and take back the activities you love — all from the comfort of your home.
✅ Safe.
✅ Proven by science.
✅ Easy to use daily.
👉 Shop our red light therapy panels today and experience lasting arthritis relief without drugs or side effects.
Check out our most popular blogs on red light therapy to save you time and money on your next purchase with Medford Red Light Therapy:
Scientific References
da Silva, R.P., Amorim, D.S., et al. (2024). The Mechanisms and Efficacy of Photobiomodulation Therapy for Arthritis: A Systematic Review. Rheumatol Int, 44(3):431–446.
Pope R.M. Apoptosis as a therapeutic tool in rheumatoid arthritis. Nat. Rev. Immunol. 2002;2:527–535. doi: 10.1038/nri846.
Firestein G.S. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed]
Pap T., Korb-Pap A. Cartilage damage in osteoarthritis and rheumatoid arthritis-two unequal siblings. Nat. Rev. Rheumatol. 2015;11:606–615. doi: 10.1038/nrrheum.2015.95. [DOI]
Carlos F.P., de Paula Alves da Silva M., de Lemos Vasconcelos Silva Melo E., Costa M.S., Zamuner S.R. Protective effect of low-level laser therapy (LLLT) on acute zymosan-induced arthritis. Lasers Med. Sci. 2014;29:757–763. doi: 10.1007/s10103-013-1413-3. [PubMed]
Stausholm, M.B., et al. (2019). Efficacy of Low-Level Laser Therapy on Pain and Disability in Knee Osteoarthritis: Meta-Analysis. BMJ Open, 9(10):e030375.
Hamblin M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. Aims Biophys. 2017;4:337–361. doi: 10.3934/biophy.2017.3.337.
Hochberg M.C. COX-2 selective inhibitors in the treatment of arthritis: A rheumatologist perspective. Curr. Top. Med. Chem. 2005;5:443–448. doi: 10.2174/1568026054201695.
Burrage P.S., Mix K.S., Brinckerhoff C.E. Matrix metalloproteinases: Role in arthritis. Front. Biosci-Landmrk. 2006;11:529–543. doi: 10.2741/1817. [DOI]
Lourinho, I., et al. (2023). Effects of Low-Level Laser Therapy in Adults with Rheumatoid Arthritis: Systematic Review and Meta-Analysis. PLOS ONE, 18(1):e0277130.
Alves A.C.A., Vieira R.d.P., Leal-Junior E.C.P., dos Santos S.A., Ligeiro A.P., Albertini R., Junior J.A.S., de Carvalho P.d.T.C. Effect of low-level laser therapy on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Arthritis Res. Ther. 2013;15:R116. doi: 10.1186/ar4296. [PubMed]
Assis L., Milares L.P., Almeida T., Tim C., Magri A., Fernandes K.R., Medalha C., Muniz Renno A.C. Aerobic exercise training and low-level laser therapy modulate inflammatory response and degenerative process in an experimental model of knee osteoarthritis in rats. Osteoarthr. Cartil. 2016;24:169–177. doi: 10.1016/j.joca.2015.07.020.
Oshima Y., Coutts R.D., Badlani N.M., Healey R.M., Kubo T., Amiel D. Effect of light-emitting diode (LED) therapy on the development of osteoarthritis (OA) in a rabbit model. Biomed. Pharmacother. 2011;65:224–229. doi: 10.1016/j.biopha.2011.02.011. [PubMed]
Bartoli D.M.F., Felizatti A.L., do Bomfim F.R.C., Bovo J.L., de Aro A.A., do Amaral M.E.C., Esquisatto M.A.M. Laser treatment of synovial inflammatory process in experimentally induced microcrystalline arthritis in Wistar rats. Lasers Med. Sci. 2021;36:529–540. doi: 10.1007/s10103-020-03055-6.
Hamblin, M.R. (2023). Can Osteoarthritis be Treated with Light? Photomed Laser Surg, 41(4):230–238.
Triumph LTD. (2020). Clinical Studies in RA: 170-Patient Photobiomodulation Trial. NCBI Clinical Summaries.
Frontiers. (2023). Current Advances of Photobiomodulation in Treating Knee Osteoarthritis. Front Cell Dev Biol, 11:128602.
Nie H., Zheng Y., Li R., Guo T.B., He D., Fang L., Liu X., Xiao L., Chen X., Wan B., et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat. Med. 2013;19:322–328. doi: 10.1038/nm.3085.
Yoshida Y., Tanaka T. Interleukin 6 and rheumatoid arthritis. Biomed. Res. Int. 2014;2014:698313. doi: 10.1155/2014/698313. [PubMed]
Dinarello C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417.
Rayegani, S.M., et al. (2022). Narrative Review: Photobiomodulation Therapy for Osteoarthritis Mechanisms. World J Orthop, 10(3):29–41.
Ball K.A., Castello P.R., Poyton R.O. Low intensity light stimulates nitrite-dependent nitric oxide synthesis but not oxygen consumption by cytochrome c oxidase: Implications for phototherapy. J. Photochem. Photobiol. B Biol. 2011;102:182–191. doi: 10.1016/j.jphotobiol.2010.12.002.
Hamblin M.R. The role of nitric oxide in low level light therapy—Art. no. 684602. In: Hamblin M.R., Waynant R.W., Anders J., editors. Mechanisms for Low-Light Therapy Iii. Volume 6846. SPIE; Bellingham, WA, USA: 2008. p. 84602.
Aud D., Peng S.L. Mechanisms of disease: Transcription factors in inflammatory arthritis. Nat. Clin. Pract. Rheum. 2006;2:434–442. doi: 10.1038/ncprheum0222.
Tascioglu, F.T., et al. (2004). LLLT Effects on KOA: RCT with 830 nm Laser. J Altern Complement Med, 10(4):685–694.
Hamblin M., Demidova T. Mechanisms of Low Level Light Therapy. Volume 6140 SPIE; Bellingham, WA, USA: 2006.
Brosseau L., Welch V., Wells G., Tugwell P., de Bie R., Gam A., Harman K., Shea B., Morin M. Low level laser therapy for osteoarthritis and rheumatoid arthritis: A metaanalysis. J. Rheumatol. 2000;27:1961–1969.
Tascioglu F., Armagan O., Tabak Y., Corapci I., Oner C. Low power laser treatment in patients with knee osteoarthritis. Swiss. Med. Wkly. 2004;134:254–258. doi: 10.4414/smw.2004.10518.
Brosseau L., Wells G., Marchand S., Gaboury I., Stokes B., Morin M., Casimiro L., Yonge K., Tugwell P. Randomized controlled trial on low level laser therapy (LLLT) in the treatment of osteoarthritis (OA) of the hand. Lasers Surg. Med. 2005;36:210–219. doi: 10.1002/lsm.20137. [PubMed]
Meireles S.M., Jones A., Jennings F., Suda A.L., Parizotto N.A., Natour J. Assessment of the effectiveness of low-level laser therapy on the hands of patients with rheumatoid arthritis: A randomized double-blind controlled trial. Clin. Rheumatol. 2010;29:501–509. doi: 10.1007/s10067-009-1347-0. [DOI]
Tomlinson R.E., Silva M.J. Skeletal Blood Flow in Bone Repair and Maintenance. Bone Res. 2013;1:311–322. doi: 10.4248/BR201304002.
Lohr N.L., Keszler A., Pratt P., Bienengraber M., Warltier D.C., Hogg N. Enhancement of nitric oxide release from nitrosyl hemoglobin and nitrosyl myoglobin by red/near infrared radiation: Potential role in cardioprotection. J. Mol. Cell. Cardiol. 2009;47:256–263. doi: 10.1016/j.yjmcc.2009.03.009.
Bülow, J., et al. (2019). LLLT Effects in Knee Osteoarthritis: Placebo-Controlled RCT. Lasers Med Sci, 34(8):1581–1588.
Katz J.N., Arant K.R., Loeser R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis A Review. JAMA-J. Am. Med. Assoc. 2021;325:568–578. doi: 10.1001/jama.2020.22171.
Charlier E., Deroyer C., Ciregia F., Malaise O., Neuville S., Plener Z., Malaise M., de Seny D. Chondrocyte dedifferentiation and osteoarthritis (OA) Biochem. Pharmacol. 2019;165:49–65. doi: 10.1016/j.bcp.2019.02.036. [DOI]
Castañeda S., Vicente E.F. Osteoarthritis: More than Cartilage Degeneration. Clin. Rev. Bone Miner. Metab. 2017;15:69–81. doi: 10.1007/s12018-017-9228-6. [DOI]
E-ARM. (2023). High‑Density LED Therapy in Hand Osteoarthritis: Pain Relief Trial. Ann Rehabil Med, 47(4):4342.
Page, M.J., Green, S., Kramer, S., et al. (2007). Electrotherapy Modalities for Adhesive Capsulitis. Cochrane Database Syst Rev, (1):CD006189.
Felson D.T. Osteoarthritis as a disease of mechanics. Osteoarthr. Cartil. 2013;21:10–15. doi: 10.1016/j.joca.2012.09.012. [PubMed]
Litwic A., Edwards M.H., Dennison E.M., Cooper C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013;105:185–199. doi: 10.1093/bmb/lds038.
Guo Q., Wang Y., Xu D., Nossent J., Pavlos N.J., Xu J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15. doi: 10.1038/s41413-018-0016-9. [DOI] [PubMed]
Smolen J.S., Landewé R.B.M., Bijlsma J.W.J., Burmester G.R., Dougados M., Kerschbaumer A., McInnes I.B., Sepriano A., van Vollenhoven R.F., de Wit M., et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020;79:685–699. doi: 10.1136/annrheumdis-2019-216655.
Lin Y.-J., Anzaghe M., Schuelke S. Update on the Pathomechanism, Diagnosis, and Treatment Options for Rheumatoid Arthritis. Cells. 2020;9:880. doi: 10.3390/cells9040880.
Avci P., Gupta A., Sadasivam M., Vecchio D., Pam Z., Pam N., Hamblin M.R. Low-Level Laser (Light) Therapy (LLLT) in Skin: Stimulating, Healing, Restoring. Semin. Cutan. Med. Surg. 2013;32:41–52. [PubMed]
Zhang R., Zhou T., Liu L., Ohulchanskyy T.Y., Qu J. Dose–effect relationships for PBM in the treatment of Alzheimer’s disease. J. Phys. D Appl. Phys. 2021;54:353001. doi: 10.1088/1361-6463/ac0740. [DOI]
de Freitas L.F., Hamblin M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016;22:348–364. doi: 10.1109/JSTQE.2016.2561201.
Chung H., Dai T.H., Sharma S.K., Huang Y.Y., Carroll J.D., Hamblin M.R. The Nuts and Bolts of Low-level Laser (Light) Therapy. Ann. Biomed. Eng. 2012;40:516–533. doi: 10.1007/s10439-011-0454-7.
Rojas J.C., Gonzalez-Lima F. Neurological and psychological applications of transcranial lasers and LEDs. Biochem. Pharmacol. 2013;86:447–457. doi: 10.1016/j.bcp.2013.06.012.
Avci P., Gupta G.K., Clark J., Wikonkal N., Hamblin M.R. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg. Med. 2014;46:144–151. doi: 10.1002/lsm.22170.
Hamblin M.R. Can osteoarthritis be treated with light? Arthritis Res. Ther. 2013;15:120. doi: 10.1186/ar4354.
Geenen R., Overman C.L., Christensen R., Asenlof P., Capela S., Huisinga K.L., Husebo M.E.P., Koke A.J.A., Paskins Z., Pitsillidou I.A., et al. EULAR recommendations for the health professional’s approach to pain management in inflammatory arthritis and osteoarthritis. Ann. Rheum. Dis. 2018;77:797–807. doi: 10.1136/annrheumdis-2017-212662.
Malmstrom V., Catrina A.I., Klareskog L. The immunopathogenesis of seropositive rheumatoid arthritis: From triggering to targeting. Nat. Rev. Immunol. 2017;17:60–75. doi: 10.1038/nri.2016.124.
Raychaudhuri S., Sandor C., Stahl E.A., Freudenberg J., Lee H.S., Jia X., Alfredsson L., Padyukov L., Klareskog L., Worthington J., et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat. Genet. 2012;44:291–296. doi: 10.1038/ng.1076.
Chen J., Wright K., Davis J.M., Jeraldo P., Marietta E.V., Murray J., Nelson H., Matteson E.L., Taneja V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016;8:43. doi: 10.1186/s13073-016-0299-7.
Fukui S., Iwamoto N., Takatani A., Igawa T., Shimizu T., Umeda M., Nishino A., Horai Y., Hirai Y., Koga T., et al. M1 and M2 Monocytes in Rheumatoid Arthritis: A Contribution of Imbalance of M1/M2 Monocytes to Osteoclastogenesis. Front. Immunol. 2017;8:1958. doi: 10.3389/fimmu.2017.01958.
Hoes J.N., Jacobs J.W.G., Buttgereit F., Bijlsma J.W.J. Current view of glucocorticoid co-therapy with DMARDs in rheumatoid arthritis. Nat. Rev. Rheumatol. 2010;6:693–702. doi: 10.1038/nrrheum.2010.179.
Sepriano A., Kerschbaumer A., Smolen J.S., van der Heijde D., Dougados M., van Vollenhoven R., McInnes I.B., Bijlsma J.W., Burmester G.R., de Wit M., et al. Safety of synthetic and biological DMARDs: A systematic literature review informing the 2019 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 2020;79:760–770. doi: 10.1136/annrheumdis-2019-216653. [DOI] [PubMed]
Osthoff A.K.R., Niedermann K., Braun J., Adams J., Brodin N., Dagfinrud H., Duruoz T., Esbensen B.A., Gunther K.P., Hurkmans E., et al. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann. Rheum. Dis. 2018;77:1251–1260. doi: 10.1136/annrheumdis-2018-213585.
Knupp M., Skoog A., Tornkvist H., Ponzer S. Triple arthrodesis in rheumatoid arthritis. Foot Ankle Int. 2008;29:293–297. doi: 10.3113/FAI.2008.0293.
Gidwani S., Fairbank A. The orthopaedic approach to managing osteoarthritis of the knee. BMJ-Brit. Med. J. 2004;329:1220–1224a. doi: 10.1136/bmj.329.7476.1220. [DOI] [PubMed]
Karu T. Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J. Photochem. Photobiol. B Biol. 1999;49:1–17. doi: 10.1016/S1011-1344(98)00219-X.
Hamblin M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018;94:199–212. doi: 10.1111/php.12864.
Karu T., Pyatibrat L., Afanasyeva N. Cellular Effects of Low Power Laser Therapy Can be Mediated by Nitric Oxide. Lasers Surg. Med. 2005;36:307–314. doi: 10.1002/lsm.20148. [DOI] [PubMed]
Paleolog E.M. Angiogenesis in rheumatoid arthritis. Arthritis Res. Ther. 2002;4:S81–S90. doi: 10.1186/ar575. [DOI]
Elshabrawy H.A., Chen Z., Volin M.V., Ravella S., Virupannavar S., Shahrara S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis. 2015;18:433–448. doi: 10.1007/s10456-015-9477-2. [PubMed]
Crofford L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013;15:S2. doi: 10.1186/ar4174. [DOI] [PubMed]
dos Santos S.A., Alves A.C.A., Leal E.C.P., Albertini R., Vieira R.D., Ligeiro A.P., Silva J.A., de Carvalho P.D.C. Comparative analysis of two low-level laser doses on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Lasers Med. Sci. 2014;29:1051–1058. doi: 10.1007/s10103-013-1467-2. [DOI]
Dos Anjos L.M.J., Salvador P.A., de Souza Á, C., de Souza da Fonseca A., de Paoli F., Gameiro J. Modulation of immune response to induced-arthritis by low-level laser therapy. J. Biophotonics. 2019;12:e201800120. doi: 10.1002/jbio.201800120. [PubMed]
Disclaimer: The Medford Red Light Therapy website is designed and intended for general informational purposes only and does not constitute the practice of medicine, nursing or other professional health care services, including the giving of medical advice, and no doctor/patient relationship is formed. The use of information on this website is at the user’s own risk. Results may vary by individual. The content of this website is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Users should not disregard or delay in obtaining medical advice for any medical condition they may have and should seek the assistance of their health care professionals for any such conditions.